Исследование противовоспалительной, анальгезирующей, ульцерогенной и антиульцерогенной активности металлокомплекса цинка производного N-изопропенилимидазола
DOI:
https://doi.org/10.18413/rrpharmacology.10.443Аннотация
Введение. Несмотря на несомненную лечебную эффективность нестероидных противовоспалительных средств, они могут вызывать серьезные побочные эффекты, из которых одним из наиболее грозных является ульцерогенное действие, влекущее за собой снижение качества жизни, а иногда и смертельные исходы. Это диктует необходимость поиска и разработки новых молекул, обладающих высокой противовоспалительной активностью, не проявляющих гастротоксического действия.
Цель работы – изучить противовоспалительную, анальгезирующую, ульцерогенную и антиульцерогенную активность металлокомплекса цинка производного N-изопропенилимидазола.
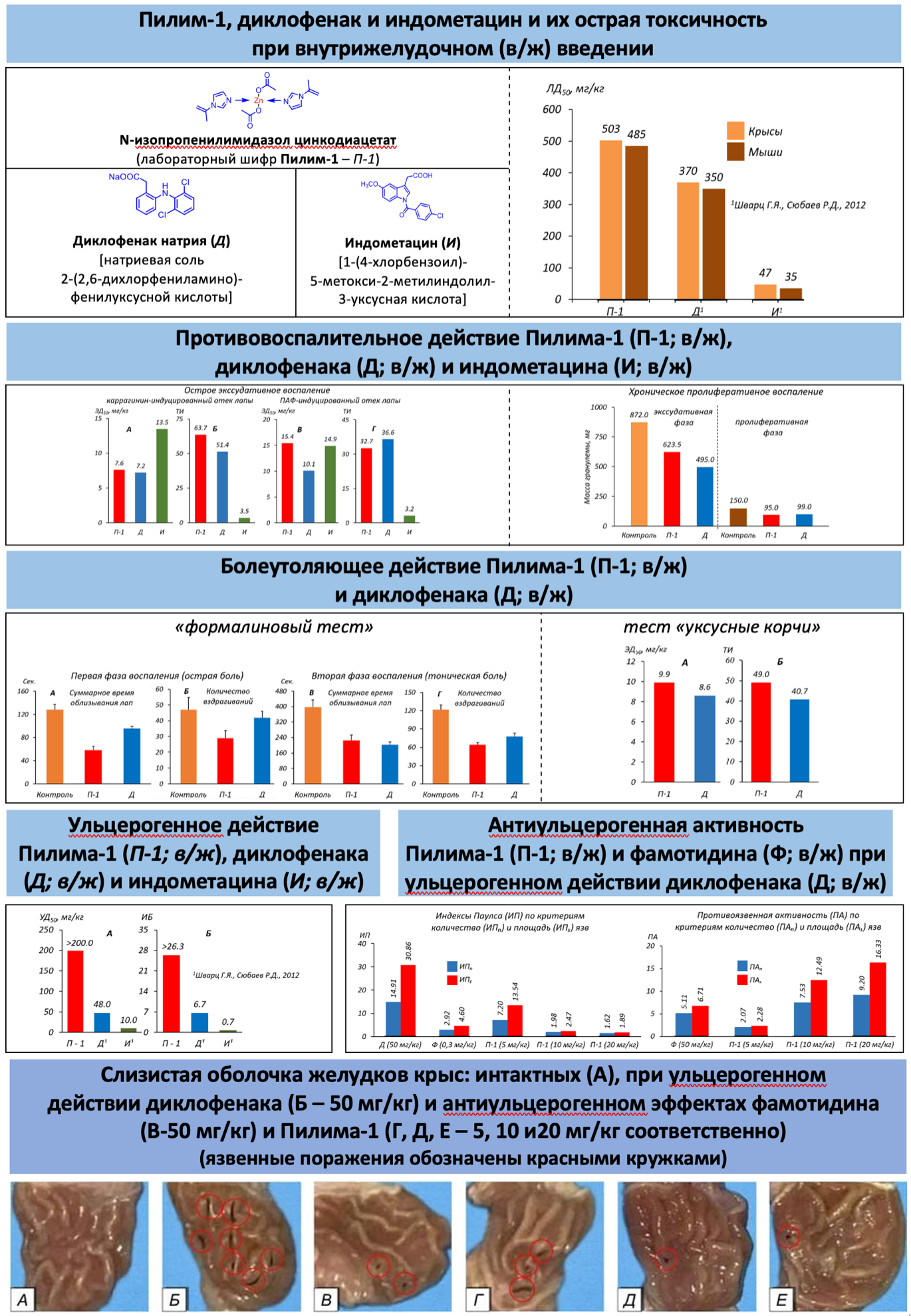
Материалы и методы. Определение средней летальной дозы – ЛД50 металлокомплекса цинка производного N-изопропенилимидазола (лабораторный шифр Пилим-1) проводили в опытах на крысах и мышах. Модели острого экссудативного и хронического пролиферативного воспаления создавали в первом случае путем субплантарного введения в заднюю лапу крыс каррагенина и полного адъюванта Фрейнда, а во втором – посредством имплантирования под кожу крыс стерильного ватного шарика («ватная гранулема»). Острую и тоническую, висцеральную и соматическую глубокую боль моделировали соответственно с помощью «формалинового теста» у крыс и теста «уксусные корчи» у мышей. Ульцерогенное и антиульцерогенное действие Пилима-1 исследовали в опытах на крысах. Пилим-1 и референс-препараты диклофенак, индометацин и фамотидин применяли внутрижелудочно.
Результаты исследований и их обсуждение. Пилим-1 менее токсичен, чем диклофенак и индометацин, обладает выраженными противовоспалительным и анальгезирующим эффектами, при этом по активности близок к диклофенаку и превосходит индометацин. По широте терапевтического действия более значим, чем диклофенак и индометацин, в отличие от последних практически не оказывает ульцерогенного действия, проявляет более значимую антиульцерогенную активность, чем фамотидин. Важную роль в механизмах противовоспалительного, анальгезирующего и гастропротекторного действия, по-видимому, играет способность Пилима-1 оказывать антигипоксическое и антиоксидантное действие, ингибирующее влияние на циклооксигеназу и 5-липооксигеназу, а также наличие в его структуре цинка и имидазола, обладающих широким спектром биологической активности.
Заключение. Сравнительно (по отношению к диклофенаку и индометацину) меньшая острая токсичность и выраженные противовоспалительный, анальгезирующий и антиульцерогенный эффекты, большая широта терапевтического действия, а также крайне низкая (практически отсутствующая) ульцерогенная активность Пилима-1 позволяют рекомендовать его для дальнейшего доклинического изучения.
Графическая аннотация
Ключевые слова:
анальгезирующая активность, антиульцерогенная активность, диклофенак, индометацин, металлокомплекс цинка производного N-изопропенилимидазола, острая токсичность, противовоспалительная активность, ульцерогенная активностьБиблиографические ссылки
Babaniyazova ZK, Rodionov IA, Babaniyazov KK (2013) Zinc medications in the regulation of redox systems. In: Current issues of correction of extremal states. Materials of the International Scientific and Practical Conference. Bryansk, pp. 108- [in Russian]
Barbarino F, Toganel E, Brilinschi C (1992) Protective effect of zinc acexamate on experimental gastric ulcers: a histochemical study. Methods and Findings in Experimental and Clinical Pharmacology 14(9): 685–694. [PubMed]
Beskhmelnitsyna EA, Kravchenko DV, Sernov LN, Dolzhikova IN, Avtina TV, Kulikov AL, Rozhnova DV, Yakushev VI, Martynov MA (2018) Search and evaluation of pharmacodynamic and pharmacokinetic parameters of selective blocker of TRPA1 ion channels from the group of substituted pyrazinopyrimidinones. Research Results in Pharmacology 4(3):49–62. https://doi.org/10.3897/rrpharmacology.4.30303
Beskhmelnitsyna EA, Pokrovskii MV, Kulikov AL, Peresypkina AA, Varavin EI (2019) Study of anti-inflammatory activity of a new non-opioid analgesic on the basis of a selective inhibitor of TRPA1 ion channels. Anti-inflammatory and Anti-Allergy Agents in Medicinal Chemistry 18(2): 110–125. https://doi.org/10.2174/1871523018666190208123700[PubMed]
Björkman RL, Hedner T, Hallman KM, Henning M, Hedner J (1992) Localization of the central antinociceptive effects of diclofenac in the rat. Brain Research 590(1–2): 66–73. https://doi.org/10.1016/0006-8993(92)91082-p [PubMed]
Bobr IS, Babaniyazov KK, Dmitrieva LA (2010) Clinical efficacy of acizol in complex treatment of chronic generalized periodontitis. Trace Elements in Medicine [Mikroelementy v Medicine] 11(1): 47–52. [in Russian]
Bondarenko DA, Dyachenko IA, Skobtsov DI, Murashev AN (2011) In vivo models of studying of analgetic activity. Biomedicine [Biomedicina] (2): 84–94. [in Russian]
Buzluma AV, Nikolaevskiy VA, Chernov YN, Slivkin AI (2017) Preclinical studies of medicinal substances. GEOTAR-MEDIA, Moscow, 384 pp. [in Russian]
Bystrova EY, Dvornikova KA, Platonova ON, Nozdrachev AD (2021) The modulating role of histamine in neuroimmune interactions. Molecular Medicine [Molecular Medicine] 19(3): 17–26. https://doi.org/10.29296/24999490-2021-03-03 [in Russian]
Chajka AV, Cheretaev IV, Khusainov DR (2015) Methods of experimental pre-clinical testing of analgesic effect of various factors on laboratory rats and mice. Scientific Notes of V.I. Vernadsky Crimean Federal University. Biology. Chemistry [Uchenye Zapiski Krymskogo Federal'nogo Universiteta Imeni V.I. Vernadskogo. Biologiya. Himiya] 1(1): 161–173. [in Russian]
de Araujo DSM, Nassini R, Geppetti P, De Logu F (2020) TRPA1 as a therapeutic target for nociceptive pain. Expert Opinion on Therapeutic Targets 24(10): 997–1008. https://doi.org/10.1080/14728222.2020.1815191 [PubMed] [PMC]
Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Zhou Q, Davies NM, Biffin JR, Regtop HL (2003) Gastrointestinal toxicity, antiinflammatory activity, and superoxide dismutase activity of copper and zinc complexes of the antiinflammatory drug indomethacin. Chemical Research in Toxicology 16(1): 28–37. https://doi.org/10.1021/tx020078o[PubMed]
Fadhil A, Qhanim H (2020) Antimicrobial studying of (Imidazole) derivative from pyrimidine. International Journal of Pharmaceutical Research 12: 913–917. https://doi.org/10.31838/ijpr/2020.12.04.128
Galenko-Yaroshevsky PA, Slavinskiy AА, Todorov SS, Popkov VL, Shelemekh OV, Lebedeva SА, Zadorozhniy AV, Zelenskaya AV, Alukhanyan LO, Nektarevskaya IB, Bunyatyan ND, Verevkin AА(2023) The effect of zinc complex of N-isopropenylimidazole on the morphological characteristics of gum tissues in experimental endodontic-periodontal lesions in rats. Research Results in Pharmacology 9(4): 1–12. https://doi.org/10.18413/rrpharmacology.9.10040.
Gammoh NZ, Rink L (2017) Zinc in infection and inflammation. Nutrients 9(6): 624. https://doi.org/10.3390/nu9060624 [PubMed] [PMC]
Gas SA, Boda FA, Roxana PR (2020) Imidazole derivatives and their antibacterial activity–a mini-review. Mini-Reviews in Medicinal Chemistry 20(11): 1380–1392. https://doi.org/10.2174/1389557520999201209213648 [PubMed]
Ghorbanzadeh B, Mansouri MT, Sahraei H, Alboghobeish S (2016) Involvement of opioid receptors in the systemic and peripheral antinociceptive actions of montelukast in the animal models of pain. European Journal of Pharmacology 779: 38–45. https://doi.org/10.1016/j.ejphar.2016.03.010 [PubMed]
Gromyko MV, Gritsuk AI (2012) Experimental models of rheumatoid arthritis. Health and Ecology [Problemy Zdorov'ya i Ekologii] 2: 115–118. https://doi.org/10.51523/2708-6011.2012-9-2-22 [in Russian]
Gurevich K, Urakov AL, Basantsev AV, Samorodov AV, Danilin AA, Purygin PP, Klenova NA, Bashirov II, Bashirova LI (2021) Synthesis of new N-mono- and N,N-dialkylated imidazole derivatives and their antiplatelet and anticoagulation activity. Pharmaceutical Chemistry Journal 55(2): 119–122. https://doi.org/10.1007/s11094-021-02395-z
Heber S, Gold-Binder M, Ciotu CI, Witek M, Ninidze N, Kress HG, Fischer MJM (2019) A Human TRPA1-specific pain model. Journal of Neuroscience 39(20): 3845–3855. https://doi.org/10.1523/JNEUROSCI.3048-18.2019 [PubMed] [PMC]
Hladkykh FV (2017) Preventive and therapeutic strategies of pharmaco-correction gastropathy induced by nonsteroidalanti-inflammatory drugs. Reviews on Clinical Pharmacology and Drug Therapy [Obzory po Klinicheskoj Farmakologii IiLekarstvennoj Terapii] 15 (4): 14–23. https://doi.org/10.17816/RCF15414-23 [in Russian]
Ho SY, Kwan YP, Qiu B, Tan A, Murray HL, Barathi VA, Tan NS, Cheung CMG, Wong TY, Wahli W, Wang X (2020) Investigating the role of PPARβ/δ in retinal vascular remodeling using pparβ/ δ-deficient mice. International Journal of Molecular Sciences 21(12): 4403.https://doi.org/10.3390/ijms21124403 [PubMed][PMC]
Horoshun MS, Lazareva AA (2022) Prescribing of non-steroidal anti-inflammatory drugs: Benefits and risks. University Therapeutic Journal [Universitetskij Terapevticheskij Vestnik] 4(1): 4–10. [in Russian]
Ivanova EA, Matyushkin AI, Vasilchuk AG, Voronina TA (2021) Effect of cyclooxygenase inhibitors etoricoxib and diclofenac sodium as well as their combinations with mexidol on behavior in rats. Vestnik Moskovskogo Universiteta. Seriya 16. Biologiya [Vestnik Moskovskogo Universiteta. Seriya 16: Biologiya] 76(3): 148–154.[in Russian]
Ivanova EA, Voronina TA (2018) Effect of diclofenac sodium on the level of histamine and serotonin in rats with acute exudative inflammation. Pharmacokinetics and Pharmacodynamics [Farmakokinetika i Farmakodinamika] 2: 12–15. https://doi.org/24411/2587-7836-2018-10009 [in Russian]
Jarosz M, Szkaradek N, Marona H, Nowak G, Młyniec K, Librowski T (2017) Evaluation of anti-inflammatory and ulcerogenic potential of zinc-ibuprofen and zinc-naproxen complexes in rats. Inflammopharmacology 25(6): 653–663. https://doi.org/10.1007/s10787-017-0361-0 [PubMed] [PMC]
Kaldybayeva АB, Praliyev KD, Sergazy A, Malmakova АЕ, Yu VK (2023) Potential of imidazole-containing derivatives for practical application (Review). Chemical Journal of Kazakhstan [Himicheskij ZHurnal Kazahstana] 2(82): 58–78. https://doi.org/10.51580/2023-2.2710-1185.14 [in Russian]
Kamchatnov PR, Chugunov AV, Chipova DT, Kazakov AYu (2023) Nonsteroidal anti-inflammatory drugs and the risk of heart failure. Russian Medical Journal [Rossijskij Medicinskij ZHurnal] 2: 88–95. [in Russian]
Karateev AE, Aleinikova TL (2016) Eicosanoids and inflammation. Modern Rheumatology Journal 10(4): 73–86. https://doi.org/10.14412/1996-7012-2016-4-73-86 [in Russian]
Karateev AE, Nasonov EL, Ivashkin VT, Martynov AI, Yakhno NN, Arutyunov GP, Alekseeva LI, Abuzarova GR, Evseev MA, Kukushkin ML, Kopenkin SS, Lila AM, Lapina TL, Novikova DS, Popkova TV, Rebrov AP, Skorobogatykh KV, Chichasova NV (2018) Rational use of nonsteroidal anti-inflammatory drugs. Clinical guidelines. Rheumatology Science and Practice [Nauchno-Prakticheskaya Revmatologiya] 56: 1–29. https://doi.org/14412/rjtao20180 48091448[in Russian]
Khnychenko LK, Okunevich IV (2015) Experimental assessment of inflammatory effect of hypoxen. Reviews on Clinical Pharmacology and Drug Therapy [Obzory po Klinicheskoj Farmakologii IiLekarstvennoj Terapii] 13(3): 35–38. https://doi.org/10.17816/RCF13335-38 [in Russian]
Kirichenko DV (2020) The effect of physic-chemical properties of the components of dosage forms on the selection of vehicle for the administration to laboratory animals. Laboratory Animals for Science 2020: 2. https://doi.org/10.29296/2618723X [in Russian]
Koba IS, Novikova EN, Ivanova EA, Yanovskaya AO, Sklyarov SP (2019) Anti-inflammatory activity of gel on the basis of chelate of zinc of the mastitis developed for treatment at cows. Agrarian Bulletin of Stavropol Region [Vestnik APK Stavropol'ya] 1(33): 39–45. [in Russian]
Korbecki J, Bobiński R, Dutka M (2019) Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflammation Research 68(6): 443–458. https://doi.org/10.1007/s00011-019-01231-1 [PubMed] [PMC]
Kuzmin AV, Zvartau EE (1998) Experimental and clinical pharmacology of analgesics. Collection of scientific papers edited by Ignatov YuD. The Pavlov First Saint Petersburg State Medical University Publishing House, Saint Petersburg, 6-15 [in Russian]
Lebedeva SA, Galenko-Yaroshevsky (Jr.) PA, Melnik SI, Kozin SV, Demura TA, Arshinov JR, Svistakova MV, Grigorevskikh EM, Galenko-Yaroshevsky PA (2022) Wound healing effect of organometallic zinc complex in the rat model of planar skin wound. Research Results in Biomedicine 8(1): 71–81. https://doi.org/10.18413/2658-6533-2022-8-1-0-5
Lebedeva SA, Galenko-Yaroshevsky PA (Jr.), Melnik SI, Kozin SV, Demura TA, Arshinov JR, Gulevskaya ON, Galenko-Yaroshevsky PA (2021) Evaluation of the wound-healing effect of an N-isopropenylimidazole zinc metal complex derivative on a linear skin wound model in rats. Pharmacy [Farmaciya] 70(6): 49–55. https://doi.org/10.29296/25419218-2021-06-09 [in Russian]
Lebedeva SA, Galenko-Yaroshevsky PA (Jr.), Rychka1 VO, Zharov YuV, Zavorina DS, Kozin SV (2022) Molecular aspects of the wound healing effect of zinc as an essential trace element. Trace Elements in Medicine [Mikroelementy v Medicine] 23(1): 14–23.https://doi.org/10.19112/2413-6174-2022-23-1-14-23 [in Russian]
Lebedeva SA, Galenko-Yaroshevsky PA (Jr.), Samsonov MYu, Erlich AB, Margaryan AG, Materenchuk MYu, Arshinov IaR, Zharov YuV, Zelenskaya AV, Shelemekh OV, Lomsadze IG, Demura TA (2023а) Molecular mechanisms of wound healing: the role of zinc as an essential microelement. Research Results in Pharmacology 9(1): 25–39. https://doi.org/10.18413/rrpharmacology.9.10003
Lebedeva SA, Galenko-Yaroshevsky PA, Fateeva TV, Pashin SS, Pashina NR, Nektarevskaya IB, Zadorozhniy AV, Shelemekh OV, Ravaeva MYu, Chuyan EN, Alukhanyan LO, Glechyan TR, Mutig K, Materenchuk MYu (2023) Effective wound healing agents based on N-alkenylimidazole zinc complexes derivatives: future prospects and opportunities. Research Results in Pharmacology 9(3): 27–39. https://doi.org/10.18413/rrpharmacology.9.10047
Litchfield Jr JT, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. Journal of Pharmacology and Experimental Therapeutics 96(2): 99–113. [PubMed]
Liu M-, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, et al. (2013) ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Reports 3: 386–400. https://doi.org/10.1016/j.celrep.2013.01.009 [PubMed] [PMC]
Liu T, Zhang L, Joo D, Sun SC (2017) Nf-kB signaling in inflammation. Signal Transduction Targeted Therapy 2: 17023. https://doi.org/10.1038/sigtrans.2017.23 [PubMed][PMC]
Mahmood A, FitzGerald AJ, Marchbank T, Ntatsaki E, Murray D, Ghosh S, Playford RJ (2007) Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut 56(2): 168-175. https://doi.org/10.1136/gut.2006.099929 [PubMed][PMC]
Maskurova YV, Gayvoronskaya TV, Sokolovsky NV (2018) Morphological characteristics of models of generalized experimental periodontitis. Treatment with acisol. Sechenov Medical Journal [Sechenovskij Vestnik] 3(33): 36–40. https://doi.org/10.26442/2218-7332_2018.3.36-40[in Russian]
Minhas D, Nidhaan A, Husni ME (2023) Recommendations for the use of nonsteroidal anti-inflammatory drugs and cardiovascular disease risk: Decades later, any new lessons learned? Rheumatic Disease Clinics of North America 49(1): 179–191. https://doi.org/10.1016/j.rdc.2022.08.006 [PubMed]
Mironov AN et al. (2012) Guidelines for pre-clinical trials of medi-cines. Part 1. Moscow: Grif and K, 944 pp. [in Russian]
Nakano Y, Arima T, Tobita Y, Uchiyama M, Shimizu A, Takahashi H (2020) Combination of peroxisome proliferator-activated receptor (ppar) alpha and gamma agonists prevents corneal inflammation and neovascularization in a rat alkali burn model. International Journal of Molecular Sciences 21(14): 5093. https://doi.org/10.3390/ijms21145093 [PubMed] [PMC]
Notova SV, Kazakova TV, Marshinskaya OV, Shoshina OV (2022) Metal-ligand forms of iron and zinc in the human body. Kazan Medical Journal [Kazanskij Medicinskij Zhurnal] 103(2): 259–268. https://doi.org/10.17816/KMJ2022-259
Novikov VE, Ilukhin SA (2013) Influence of hypoxen on acetylsalicylic acid efficiency in acute inflammation. Experimental and Clinical Pharmacology [Éksperimentalnaya i Klinicheskaya Farmakologiya] 76(4): 32–35. [PubMed]
Novikov VE, Krukova NO, Novikov AS (2010) Gastroprotective properties of mexidol and hypoxen. Experimental and Clinical Pharmacology [Éksperimentalnaya i Klinicheskaya Farmakologiya] 73(5): 15–18. [PubMed]
Ohkawara T, Nishihira J, Nagashima R, Takeda H, Asaka M (2006) Polaprezinc protects human colon cells from oxidative injury induced by hydrogen peroxide: relevant to cytoprotective heat shock proteins. World Journal of Gastroenterology 12(38): 6178-6181. https://doi.org/10.3748/wjg.v12.i38.6178 [PubMed] [PMC]
Palamar AA, Yaremiy IN, Chornous VA, Grozav AN, Vovk MV (2020) Effect of [(1-Phenyl-5-formyl-1H-imidazole-4-yl)thio]acetic acid on the antioxidant status of rat liver and kidneys under conditions of carbon tetrachloride intoxication. Pharmaceutical Chemistry Journal [Himiko-Farmacevticheskij ZHurnal] 54(11): 17–21. https://doi.org/10.30906/0023-1134-2020-54-11-17-21 [in Russian]
Panasenko LM, Kartseva TV, Nefedova ZhV, Zadorina-Khutornaya EV (2018) Role of the main mineral substances in the child nutrition. Russian Bulletin of Perinatology and Pediatrics [Rossiyskiy Vestnik Perinatologii i Pediatrii] 63(1): 122–127. https://doi.org/21508/1027-4065-2018-63-1-122-127 [in Russian]
Patel JA, Patel NB, Maisuriya PK, Tiwari MR, Purohit AC (2022) Structure activity design, synthesis and biological activity of newer imidazole-triazine clubbed derivative as antimicrobial and antitubercular agents. Letters in Organic Chemistry 19(2): 126–134. https://doi.org/10.2174/1570178618666210521150011
Pham CT, Nguyen V, Choi Y, Kim D, Jung O, Lee DJ, Kim HJ, Lee MW, Yoon J, Kim HM, Lee S (2021) Hypochlorite-activated fluorescence emission and antibacterial activities of imidazole derivatives for biological applications. Frontiers in Chemistry 9: https://doi.org/10.3389/fchem.2021.713078 [PubMed] [PMC]
Podobed VM (2015) Zinc Сarnosin: a new formula of gastroprotection and making up zinc deficiency. Medical news [Meditsinskie Novosti] 2: 17–20. [in Russian]
Pogilova EV (2014) Influence of antihypoxants on the developmentof carrageenin-induced inflammation. I.P. Pavlov Russian Medical Biological Herald [Rossijskij Mediko-Biologicheskij Vestnik Imeni Akademika I. P. Pavlova] 4: 61–67. [in Russian]
Rajam S, Dileepan B, Maruthamuthu B (2020) Spectral characterization and biological activity of newly synthesized imidazole derivative. World Journal of Pharmacy and Pharmaceutical Sciences 4(4): 887–897. https://www.researchgate.net/publication/340931576
Serebrennikova SN, Seminsky IZh, Guzovskaiia EV, Gutsol LO (2023) Inflammation as a fundamental pathological process: lecture 2 (cellular component). Baikal Medical Journal [Bajkal'skij Medicinskij ZHurnal] 2(2): 65–76. https://doi.org/10.57256/2949-0715-2023-2-65-76 [in Russian]
Shakhmardanova SA, Galenko-Yaroshevsky PA (2015) N-alkenylimidazole metal complex derivatives. Antihypoxic properties, modes of action, prospects for clinical use. Prosveshchenie-Yug, Krasnodar, 276 pp. [in Russian]
Shakhmardanova SA, Galenko-Yaroshevsky PA (2016) Zinc complex compound with N-alkenylimidazoles: biological activity and application in medicine. Sechenov Medical Journal [Sechenovskij Vestnik] 3(25): 84–90. [in Russian]
Shvarts GYa, Subaev RD (2012) Methodological recommendations for the preclinical study of nonsteroidal anti-inflammatory drugs. In: Mironov AN (Ed.) Guidelines for pre-clinical trials of medicines. GrifandK, Moscow, 746–759 pp. [in Russian]
Skrovanek S, DiGuilio K, Bailey R, Huntington W, Urbas R, Mayilvaganan B, Mercogliano G, Mullin JM (2014) Zinc and gastrointestinal disease. World Journal of Gastrointestinal Pathophysiology 15(4): 496–513. https://doi.org/10.4291/wjgp.v5.i4.496 [PubMed] [PMC]
Stone WL, Basit H, Burns B (2022) StatPearls (Pathology, Inflammation) [Internet]. Treasure Island (FL), StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK534820/ [PubMed]
Tobita Y, Arima T, Nakano Y, Uchiyama M, Shimizu A, Takahashi H (2020) Peroxisome proliferator-activated receptor beta/delta agonist suppresses inflammation and promotes neovascularization. International Journal of Molecular Sciences 21(15): 5296. https://doi.org/10.3390/ijms21155296 [PubMed] [PMC]
Tobita Y, Arima T, Nakano Y, Uchiyama M, Shimizu A, Takahashi H (2021) Effects of selective peroxisome proliferator activated receptor agonists on corneal epithelial wound healing. Pharmaceuticals 14(2): 88. https://doi.org/10.3390/ph14020088 [PubMed] [PMC]
Turgeneva LB, Novikov VE, Pogilova EV (2011) Treatment of inflammatory periodontal diseases with mexidol. Pathogenesis [Patogenez] 9(3): 67. [in Russian]
Valls A, Andreu JJ, Falomir E, Luis SV, Atrian-Blasco E, Mitchell SG (2020) Imidazole and imidazolium antibacterial drugs derived from amino acids. Pharmaceuticals 13(12): https://doi.org/10.3390/ph13120482 [PubMed] [PMC]
Velts NY, Zhuravleva EO, Bukatina TM, Kutekhova GV (2018) Nonsteroidal anti-inflammatory drugs: problems of safe use. Safety and Risk of Pharmacotherapy [Bezopasnost' i Risk Farmakoterapii] 6(1): 11–18. https://doi.org/10.30895/2312-7821-2018-6-1-11-18 [in Russian]
Vidhya MR, Malar SJ (2020) Imidazole derivatives–potential compounds with antimicrobial and antioxidant efficacy. International Journal of Advanced Science and Technology 29(5): 183–189.
Voelkl J, Tuffaha R, Luong TT D, Zickler D, Masyout J, Feger M, Verheyen N, Blaschke F, Kuro-O M, Tomaschitz A, Pilz S, Pasch A, Eckardt KU, Scherberich JE, Lang F, Pieske B, Alesutan I (2018) Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. Journal of the American Society of Nephrology 29(6): 1636–1648. https://doi.org/10.1681/ASN.2017050492 [PubMed] [PMC]
Voronina ТА, Guzevatykh LS (2012) Methodological recommendations for the study of the analgesic activity of medicines. In: Mironov AN (Ed.) Guidelines for pre-clinical trials of medicines. Grif and K, Moscow, 197–219. [in Russian]
Wheeler-Aceto H, Cowan A (1991) Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology 104: 35–44. https://doi.org/10.1007/BF02244551[PubMed]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2024 Pavel A. Galenko-Yaroshevsky, Olga V. Shelemekh, Viktor L. Popkov, Andrey V. Zadorozhniy, Irina B. Nektarevskaya, Natalia D. Bunyatyan, Roman A. Murashko, Svetlana А. Lebedeva, Anait V. Zelenskaya, Aleksandr V. Uvarov, Olga N. Gulevskaya, Lusine O. Alukhanyan, Tereza R. Glechyan, Alina V. Sergeeva, Alina V. Kornetskaya, Nikolay E. Korovaykin

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

