Effect of hydrogel with a new acexamic acid derivative on the survival of ischemic tissue
DOI:
https://doi.org/10.18413/rrpharmacology.11.864Аннотация
Introduction: Prevention and correction of the consequences of local ischemia developing during various surgical interventions is a pressing issue in modern medicine.
Materials and Methods: The study was conducted on 24 Agouti viable yellow mice weighing 30-35 g. An ischemic skin flap measuring 10 x 30 mm was created on the back skin of mice with a mutation in the Agouti viable yellow gene. Immediately after the procedure, the animals were topically treated twice daily with a hydrogel containing 2-ethyl-6-methyl-3-hydroxypyridinium N-acetyl-6-aminohexanoate (2E-6M-3G-NA-6A) (5%) and reference drug, panthenol gel (10%) for 10 days. Wound healing was monitored using planimetry, microcirculation, and histomorphological and biochemical parameters.
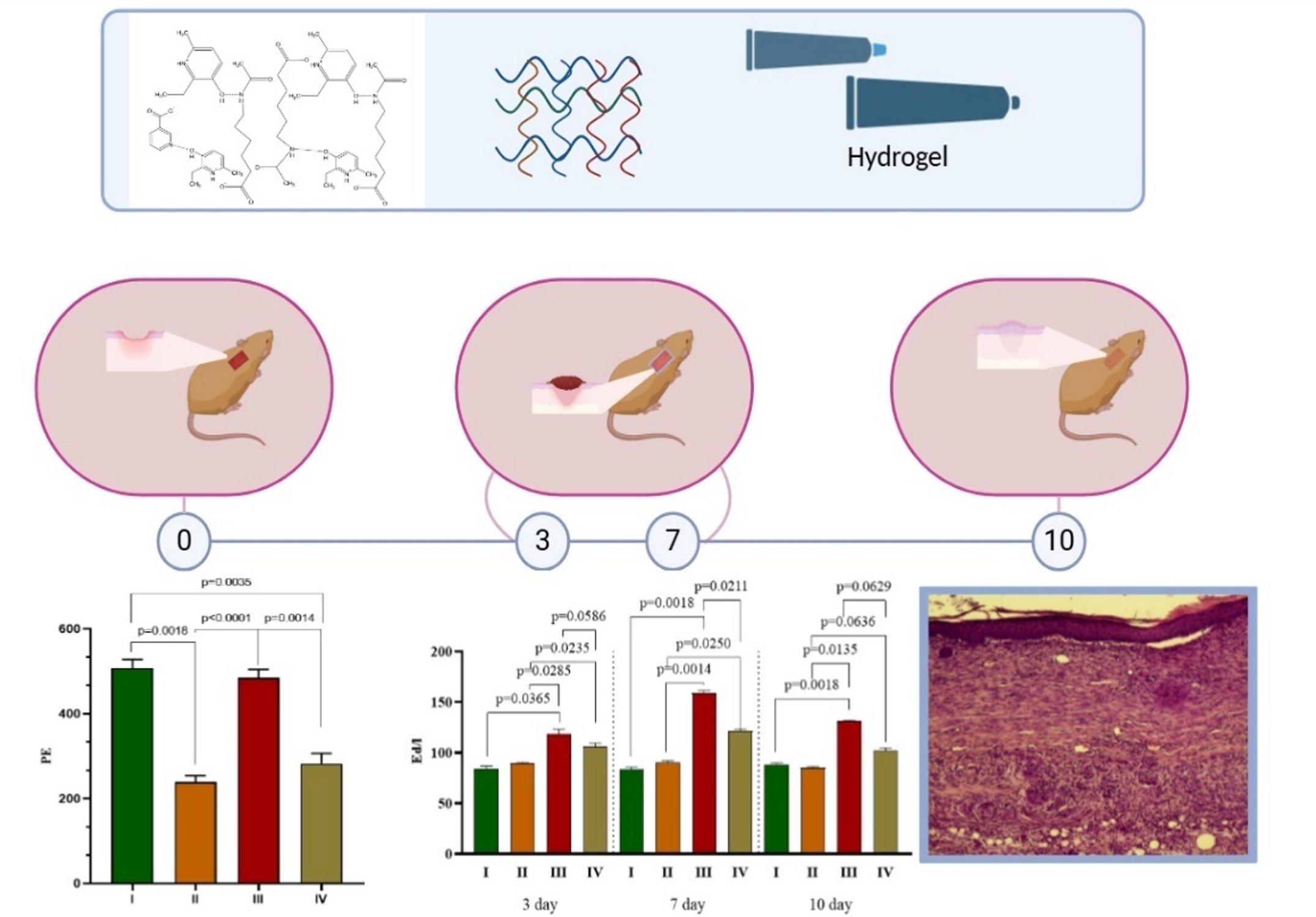
Results: The efficacy of a hydrogel based on 2E-6M-3G-NA-6A on skin tissue reparation processes was studied in transgenic C57BL/6J mice with a dominant mutation in the Agouti yellow (Au/a) gene using an isolated pedicle skin flap model. Topical application of 5% hydrogel based on (2E-6M-3G-NA-6A) twice daily for 10 days resulted in a 1.6-fold decrease in the area of tissue necrosis compared to that in the control group without pharmacological support. On the 10th day of the experiment, in the group of animals treated with the hydrogel based on 2E-6M-3G-NA-6A, an increase in the level of microcirculation was observed on average to 489.1 ± 29.1 perfusion units (PU) (p = 0.0001) compared to such in the group of animals without treatment – 242.3 ± 19.6 PU (p < 0.0018). Hydrogel based on 2E-6M-3G-NA-6A, when applied topically to an ischemic wound, led to an increase in the level of alkaline phosphatase on the 7th day to values of 157.4 ± 12.7 (p < 0.0014). U/L, which is 1.8 times higher than in the control group.
Conclusion: 2E-6M-3G-NA-6A in a hydrogel dosage form is capable of stimulating wound healing in ischemic tissues to the level of non-ischemic wounds and may become a new and effective method for treating long-term non-healing wounds.
Графическая аннотация
Ключевые слова:
hydrogel, 2-ethyl-6-methyl-3-hydroxypyridinium N-acetyl-6-aminohexanoate, Agouti yellow, Carbopol 940Библиографические ссылки
Blinova E, Pakhomov D, Shimanovsky D, Kilmyashkina M, Mazov Y, Demura T, Drozdov V, Blinov D, Deryabina O, Samishina E, Butenko A, Skachilova S, Sokolov A, Vasilkina O, Alkhatatneh BA, Vavilova O, Sukhov A, Shmatok D, Sorokvasha I, Tumutolova O, Lobanova E (2021) Cerium-containing N-acetyl-6-aminohexanoic acid formulation accelerates wound reparation in diabetic animals. Biomolecules 11(6): 834. https://doi.org/10.3390/biom11060834 [PubMed] [PMC]
Danilenko AP, Khentov AA, Korokin MV, Wan Sun, Kuznetsov AV, Gudyrev OS, Pokrovsky MV, Chao Zhu, Teplyakov MY, Nazarmamadov HE, Taran EI, Kochkarov AA, Kalinina EA, Kozlova KR, Tarasov AA, Milashenko VN, Lebedev PR, Danilenko LM (2025) Efficacy of 2-ethyl-6-methyl-3-hydroxypyridinium N-acetyl-6-aminohexanoate complex for pharmacological correction of bone tissue in transgenic mice lacking 11β-hydroxysteroid dehydrogenase type 2 expression. Experimental and Clinical Pharmacology [Eksperimental’naya i Klinicheskaya Farmakologiya] 88(3): 29-35. https://colab.ws/articles/10.30906%2F0869-2092-2025-88-3-29-35# [in Russian]
Danilenko AP, Khentov AA, Kuznetsov AV, Shmigerova VS, Stepenko YuV, Tarasova AP, Yakushev VI, Boeva EV, Miller AV, Tatarenkova IA, Puzanova TV, Loboda YaV, Trashchenko AP, Gudyrev OS, Motailo AL, Kuzichkin VN, Milashechko VN, Danilenko LM (2025) Predictive analysis and prediction of the main molecular targets for the N-acetyl-6-aminohexanoate derivative. Research Results in Pharmacology 11(2): 73–85. https://doi.org/10.18413/rrpharmacology.11.560
Danilenko AP, Zhunusov NS, Khentov AA, Pokrovsky VM, Kuznetsov AV, Kulikov AL, Nadezhdin SV, Danilenko LM, Peresypkina AA, Loboda YaV, Gudyrev OS, Rubanov MYu, Avtina TV (2024) Pharmacokinetic properties of a new supramolecular complex based on 2-ethyl-6-methyl-3-hydroxypyridinium N-acetyl-6-aminohexanoate and 2-ethyl-6-methyl-3-hydroxypyridinium 3-pyridinocarbonoate, which has osteoprotective activity in in vitro Experimental and Clinical Pharmacology [Eksperimental’naya i Klinicheskaya Farmakologiya] 87(2): 13–19. https://doi.org/10.30906/0869-2092-2024-87-2-13-19 [in Russian]
Darvishi S, Tavakoli S, Kharaziha M, Girault HH, Kaminski CF, Mela I (2022) Advances in the sensing and treatment of wound biofilms. Angewandte Chemie International Edition 61(13): e202112218 https://doi.org/10.1002/anie.202112218 [PubMed] [PMC]
Dixon D, Edmonds М (2021) Managing diabetic foot ulcers: Pharmacotherapy for wound healing. Drugs 81(1): 29-56. https://doi.org/10.1007/s40265-020-01415-8 [PubMed]
Gora IM, Ciechanowska A, Ladyzynski P (2021) NLRP3 inflammasome at the interface of inflammation, endothelial dysfunction, and type 2 diabetes. Cells 10(2): 314 https://doi.org/10.1089/ars.2023.0524[PubMed] [PMC]
Kolesnik IM, Pokrovsky MV, Lazarenko VA, Gudyrev OS, Gureev VV, Danilenko LM, Korolev AE, Narykov RA, Zheludkov IS, Lopatin DV, Egorova EO, Alehin SA (2010) Remote preconditioning influence on ischemic tissues survival potential. Journal of Experimental and Clinical Surgery [Vestnik Eksperimentalnoy i Klinicheskoy Khirurgii] 3(3): 214–217. [in Russian]
Kostina DA, Shcheblykina OV, Peresypkina AA, Molchanov VV, Arkhipov VV, Zhernakova NI, Simokhina VS, Zhu C, Gudyrev OS (2025) Assessment of the severity of delayed changes in the state of the neuromuscular system during correction of local cold injury of III-IV degree with pCMV-VEGF165 plasmid preparation in an experiment. Research Results in Pharmacology 11(1): 90–98. https://doi.org/10.18413/rrpharmacology.11.550
Mirhaj M, Labbaf S, Tavakoli M, Seifalian AM (2022) Emerging treatment strategies in wound care. International Wound Journal 19(7): 1934–1954.| https://doi.org/10.1111/iwj.13786[PubMed] [PMC]
Molnar A, Magyar Z, Nachmias DB, Mann D, Szabo B, Toth L, Nemeth N. (2020) Effect of short-term ischemia on microcirculation and wound healing of adipocutaneous flaps in the rat Acta Cirúrgica Brasileira 34(12): e201901203. https://doi.org/10.1590/s0102-865020190120000003 [PubMed] [PMC]
Morgun ЕI, Rogovaya ОS, Vorotelyak ЕА (2018) Ischemic non-healing skin wound model: cell death and wound healing mechanisms. Modern Technologies in Medicine [Sovremennye Tekhnologii v Medicine] 10(4): 69–77. https://doi.org/10.17691/stm2018.10.4.08 [in Russian]
Pakhomov DV, Blinova EV, Shimanovsky DN, Kilmyashkina MF, Kazaeva MA, Blinov DS, Nelipa MV, Nikolaev AV, Alkhatatnekh BA, Skachilova SYa, Bogoyavlenskaya TA, Kytko OV (2020) Evidence-based aspects of stimulating uncomplicated wound healing with local application of silver salt of acexamic acid. Operative Surgery and Clinical Anatomy [Operativnaya Khirurgiya i Klinicheskaya Anatomiya] 4(1): 19–25. https://doi.org/10.17116/operhirurg2020401119 [in Russian]
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019). Wound healing: a cellular perspective. Physiological Reviews 99: 665–706. https://doi.org/10.1152/physrev.00067.2017 [PubMed] [PMC]
Terekhov AG, Pankrusheva TA, Chekmareva MS, Mishina ES, Zaitsev AI, Borzenkov AD, Tokmakov AD, Grigoryan AY (2024) Evaluation of the effectiveness of benzalkonium chloride, dexpanthenol and pentoxifylline combination in the local treatment of skin wounds in ischemic conditions (experimental study). Journal of New Medical Technologies [Vestnik Novykh Meditsinskikh Tekhnologiy] 31(2):10–13. https://doi.org/10.24412/1609-2163-2024-2-10-13 [in Russian]
Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B (2020) Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 12(8): 735. https://doi.org/10.3390/pharmaceutics12080735 [PubMed] [PMC]
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, Dennis VA, Singh SR (2017) Advances in skin regeneration using tissue engineering. International Journal of Molecular Sciences 18(4): 789 https://doi.org/10.3390/ijms18040789 [PubMed] [PMC]
Wan R, Weissman JP, Grundman K, Lang L, Grybowski DJ, Galiano RD (2021) Diabetic wound healing: the impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Repair and Regeneration 29(4): 573-581. https://doi.org/10.1111/wrr.12954 [PubMed]
Wang D, Yin Y, Wang S, Zhao T, Gong F, Zhao Y, Wang B, Huang Y, Cheng Z, Zhu G, Wang Z, Wang Y, Ren J, Liang G, Li X, Huang Z (2025) FGF1(deltaHBS) prevents diabetic cardiomyopathy by maintaining mitochondrial homeostasis and reducing oxidative stress via AMPK/Nur77 suppression. Signal Transduction and Targeted Therapy 6(1): 133. https://doi.org/10.1038/s41392-021-00542-2 [PubMed] [PMC]
Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, Luo S, Li Z, Liu P, Han J, Harding IC, Ebong E E, Cameron SJ, Stewart AG, Weng J (2021) Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacological Reviews 73(3): 924–967 https://doi.org/10.1124/pharmrev.120.000096[PubMed]
Yan C, Chen J , Wang C , Yuan M , Kang Y, Wu Z, Li W, Zhang G, Machens H-G, Rinkevich Y, Chen Z, Yang X, Xu X (2022) Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Angewandte Chemie International Edition 61(13): e202112218 https://doi.org/10.1002/anie.202112218 [PubMed] [PMC]
Yang DR, Wang MY, Zhang CL, Wang Y (2024) Endothelial dysfunction in vascular complications of diabetes: a comprehensive review of mechanisms and implications. Frontiers in Endocrinologt (Lausanne) 5(15): 1359255. https://doi.org/10.3389/fendo.2024.1359255 [PubMed] [PMC]
Zhang H, Yan Z, Zhu J, Li Z, Chen L, Zheng W, Dai Z, Yang J, Yun X, Wang Y, Zhou H, Jiang Z, Yu Q, Li S, Huang W, Yang L (2025) Extracellular mitochondrial-derived vesicles affect the progression of diabetic foot ulcer by regulating oxidative stress and mitochondrial dysfunction. Advanced Science 12(10): e2407574. https://doi.org/10.1002/advs.202407574[PubMed] [PMC]
Zhang M, Wang G, Wang D, Zheng Y, Li Y, Meng W, Zhang X, Du F, Lee S (2021) Ag@ MOF-loaded chitosan nanoparticle and polyvinyl alcohol/sodium alginate/chitosan bilayer dressing for wound healing applications. International Journal of Biological Macromolecules 175: 481-494. https://doi.org/10.1016/j.ijbiomac.2021.02.045 [PubMed]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2025 Khentov AA, Kuznetsov AV, Bolgov AA, Danilenko AP, Miller AV, Zhilyakova ET, Yurov SM, Bratchin KA, Puzanova TV, Loboda YV, Yurova PM, Avtina NV, Danilenko LM

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

