The evaluation of pharmacokinetic parameters of 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide and its metabolites in rat plasma
DOI:
https://doi.org/10.18413/rrpharmacology.10.523Abstract
Introduction: 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide (ODASA) is a new pharmacologically activecompound, which is capable of reducing intraocular pressure by inhibiting carbonic anhydrase II. It is necessary to calculate the pharmacokinetic parameters of this compound and its metabolites in blood plasma of laboratory animals during a preclinical study. Aim: Evaluation of pharmacokinetic parameters of ODASA and its metabolites in rat bloodplasma after instillation of ocular suspension and its intraperitoneal administration.
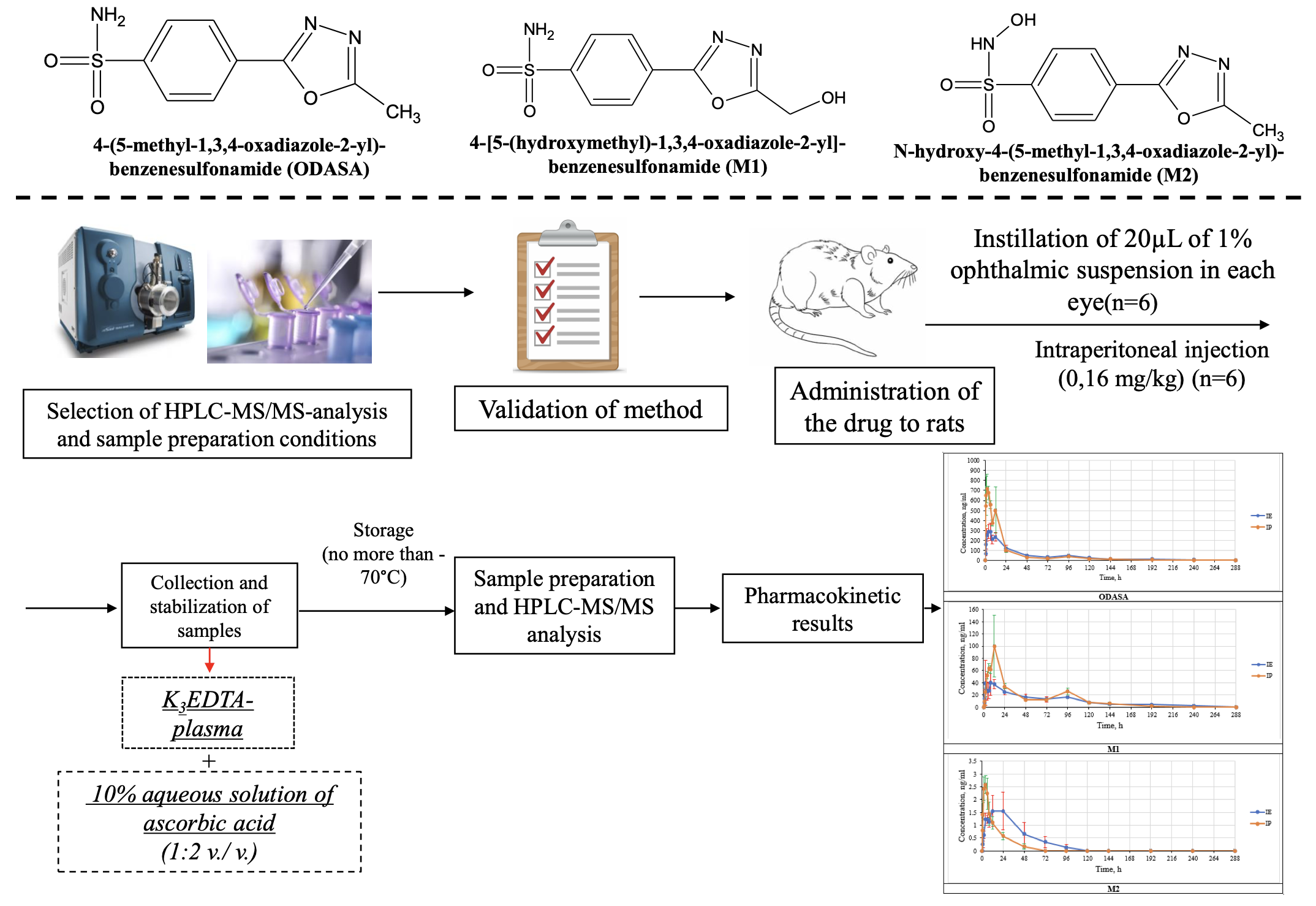
Materials and Methods: The study was conducted on 2 groups of Wistar rats, each including 6 individuals. The firstgroup underwent instillation of 1% ocular suspension of ODASA of 20 µL into each eye (1.6 mg/kg). The drug was injected intraperitoneally at the same dose to the second group. Blood sampling was performed before the administration,0.5h, 1h, 2h, 4h, 6h, 8h, 12h, 24h, 48h, 72 h, 96h, 120h, 144h, 192h, 240h, 288h after the administration. Plasma was immediately stabilized by 10% solution of ascorbic acid and frozen to a temperature no higher than -70°C. The sampleswere analyzed using the HPLC-MS/MS method. Chromatographic separation was performed on a Kinetex Phenyl Hexylcolumn (50*4.6 mm, 2.6 microns) in a gradient mode. Detection was carried out in the MRM mode using electrosprayionization.
Results: The developed method was validated in the range of 2-2000 ng/mL for ODASA and 4-[5-(hydroxymethyl)-1,3,4-oxadiazole-2-yl]-benzenesulfonamide (M1) and 0.5 -500.0 ng/mL for N-hydroxy-4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide (M2). The size of the maximum plasma concentration after instillation of ODASA into eyes was 349.85±62.50 ng/mL, in M1 – 30.91±6.00 ng/mL and in M2 – 2.70±0.62 ng/mL (M±SEM). The half-life time of ODASA after ocular administration was 46.4±3.8 h, M1 – 70.0±14.3 h, and M2 - 36.5±15.2 h (M±SEM). The relative bioavailability of ODASA compared with injection was 81.03%.
Conclusion: The performed validation of the method guaranteed the accuracy of the obtained results. The activesubstance has a high relative bioavailability after instillation into eyes. M1 is a major metabolite, and M2 is a minormetabolite. The studied compounds had a long half-life.
Graphical Abstract
Keywords:
HPLC-MS/MS, plasma, stabilization, validation, pharmacokinetics, bioavailability, rats, carbonic anhydrase II inhibitor, N-hydroxysulfonamideReferences
Begou O, Drabert K, Theodoridis G, Tsikas D (2020) GC-NICI-MS analysis of acetazolamide and other sulfonamide (R-SO2-NH2) drugs as pentafluorobenzyl derivatives [R-SO2-N(PFB)2] and quantification of pharmacological acetazolamide in human urine. Journal of Pharmaceutical Analysis 10(1): 49–59. https://doi.org/10.1016/j.jpha.2019.11.006[PubMed] [PMC]
Dhandar AG, Chaudhari SR, Ganorkar SB, Patil AS, Surana SJ (2022) Mini-review on bioanalytical estimation of brinzolamide. Current Pharmaceutical Analysis 18(3): 265–272. https://doi.org/10.2174/1573412917666210812103414
ICH guideline M10 on bioanalytical method validation and study sample analysis (2022) https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf. (access date: 16.08.2024)
Khokhlov AL, Yaichkov II, Korsakov MK, Shetnev AA, Volkhin NN, Petukhov SS (2023) Development of quantification methods of a new selective carbonic anhydrase II inhibitor in plasma and blood and study of the pharmacokinetics of its ophthalmic suspension in rats. Research Results in Pharmacology 9(4): 53–64. https://doi.org/10.18413/rrpharmacology.9.10056
Khokhlov AL, Yaichkov II, Panova VA, Efimova YA, Shetnev AА, Ivanovsky SA, Korsakov MK, Volkhin NN, Petukhov SS (2024) Identification and synthesis of metabolites of 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide. Research Results in Pharmacology 10(4): 15–27. https://doi.org/10.18413/rrpharmacology.10.498
Lo Faro AF, Tini A, Gottardi M, Pirani F, Sirignano A, Giorgetti R, Busardò FP (2021) Development and validation of a fast ultra-high-performance liquid chromatography tandem mass spectrometry method for determining carbonic anhydrase inhibitors and their metabolites in urine and hair. Drug Testing and Analysis 13(8): 1552–1560. https://doi.org/10.1002/dta.3055 [PubMed] [PMC]
Malygin AS, Popov NS, Demidova MA, Shatokhina NA (2020) Development and validation of HPLC-MS/MS method of determination of a new derivative of valproleic acid and 1,3,4-thiadiazole in rabbit blood plasma for pharmacokinetic study. Problems of Biological, Medical, and Pharmaceutical Chemistry [Voprosy Biologhicheskoi, Meditsinskoi i Farmatsevticheskoi Khimii] 23(8): 26–33. https://doi.org/10.29296/25877313-2020-08-04 [in Russian]
Mironov AN (ed.) (2012) Guidelines for Conducting Preclinical Studies of Medicines. Volume 1. Polygraph Plus, Moscow, 944 pp.
On Approval of the Rules for Conducting Bioequivalence Studies on Medicines in the Eurasian Economic Union. Decision of the Council of the Eurasian Economic Commission № 85 of November 3 (2016) http://docs.cntd.ru/document/456026107 (access date: 16.08.2024).
Xiong J, Xu Y, He S, Zhang Y, Wang Z, Wang S, Jiang H (2020) Pharmacokinetics and bioavailability of tildipirosin in rabbits following single-dose intravenous and intramuscular administration. Journal of Veterinary Pharmacology and Therapeutics. 43(5): 448–453. https://doi.org/10.1111/jvp.12882 [PubMed]
Yaichkov II, Korsakov MK, Shetnev AA, Volkhin NN, Petukhov SS (2024) Development and validation of the method of quantification of 5-[5-(trifluoromethyl)-1,2-oxazole-3-yl]-furan-2-sulfonamide and its metabolites in laboratory animal plasma. Drug Development & Registration [Razrabotka i Registratsiya Lekarstvennykh Sredstv] 13(3): 219–230. https://doi.org/10.33380/2305-2066-2024-13-3-1771 [in Russian]
Wilson S E, Carpenter J W, Gardhouse S, KuKanich B (2023) Pharmacokinetics of mavacoxib in New Zealand White rabbits (Oryctolagus cuniculus). American Journal of Veterinary Research. 84 (5): 1–5. https://doi.org/10.2460/ajvr.22.11.0196 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Khokhlov AL, Yaichkov II, Shetnev AA, Korsakov MK, Volkhin NN, Petukhov SS, Tyushina AN, Lasaryanz OE

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

