Novel derivative of nicotinic acid ameliorates doxorubicin-induced cardiac injury via regulation of redox homeostasis
DOI:
https://doi.org/10.18413/rrpharmacology.10.514Аннотация
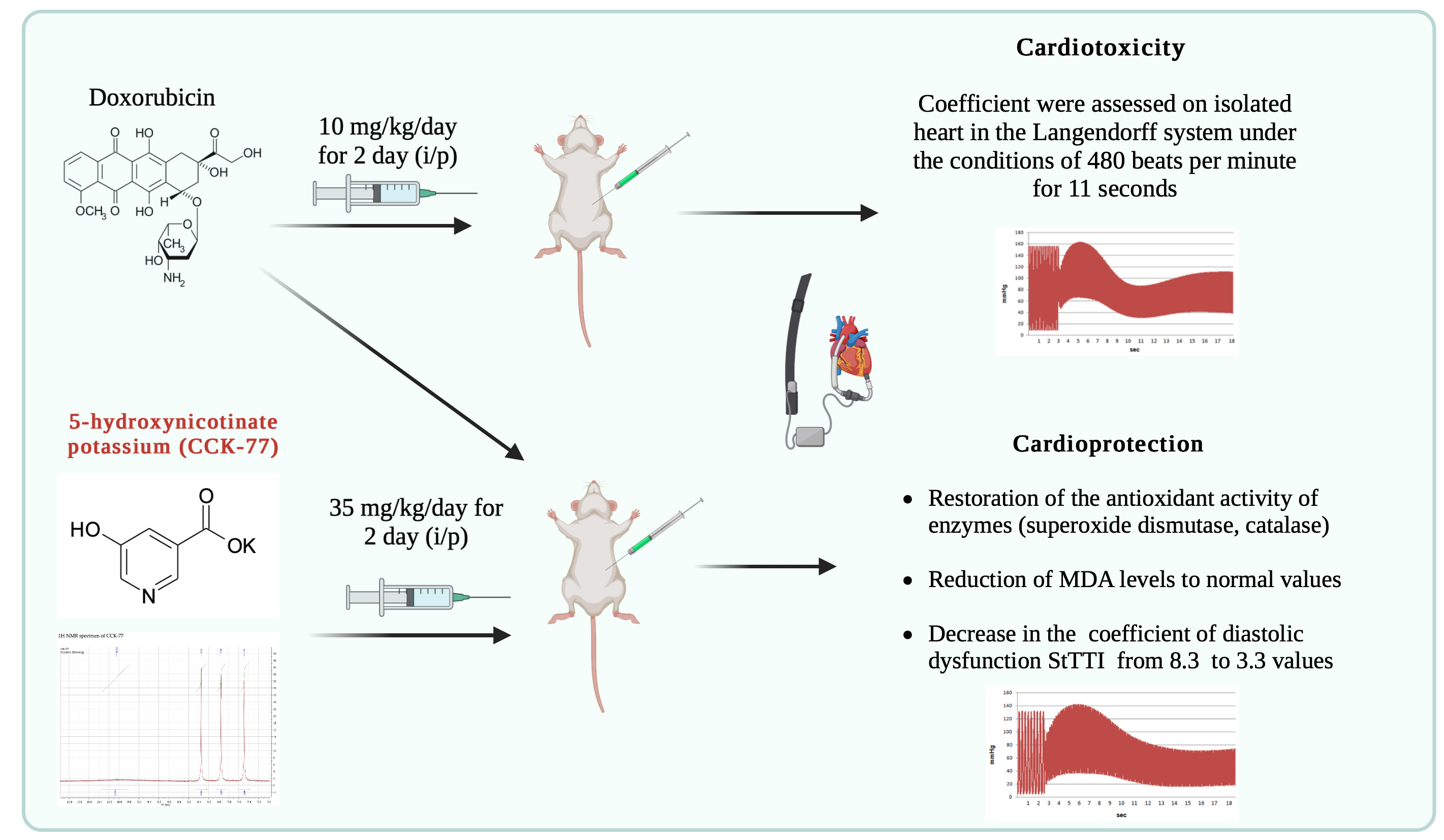
Introduction: Doxorubicin (DOX) is an anthracycline antibiotic with considerable significance in clinics as an anticancer agent, which is limited by its cardiotoxicity, though. A large number of possible therapeutic strategies for reducing cardiotoxicity with doxorubicin have been studied. However, none of them fully meets the requirements of clinical practice. The aim of the study: to evaluate the cardioprotective effects of a new original heterocyclic compound, a pyridine-3-carboxylic acid derivative potassium 5-hydroxynicotinate.
Materials and Methods: Cardiac injury was induced by intraperitoneal administration of doxorubicin (DOX) at a dose of 20 mg/kg. After 48 hours, the parameters of left ventricular contractility and StTTI coefficient were assessed on isolated heart in the Langendorff system under the conditions of 480 beats per minute for 11 seconds. Additionally, specific markers of myocardial injury were determined. The lipid peroxidation products and SOD activity were measured as well to challenge whether the compound is able to reduce oxidative stress.
Results: The study showed that pretreatment by potassium 5-hydroxynicotinate (35 mg/kg, 48 h) attenuated DOX-induced damage, resulting in a significant decrease in the StТТI coefficient to 3.3 values and in the restoration of the antioxidant activity of enzymes.
Conclusion: The obtained data totally demonstrate the protective effects of potassium 5-hydroxynicotinate in DOX-induced cardiomyopathy. A significant role in potassium 5-hydroxynicotinate-mediated cardioprotection is, apparently, related to the reduction of oxidative stress and down-regulation of the level of intracellular calcium.
Графическая аннотация
Ключевые слова:
potassium 5-hydroxynicotinate, doxorubicin, coefficient StТТI, oxidative stress, Wistar ratsБиблиографические ссылки
Adeyemi DH, Obembe OO, Hamed MA, Akhigbe RE (2024) Sodium acetate ameliorates doxorubicin-induced cardiac injury via upregulation of Nrf2/HO-1 signaling and downregulation of NFkB-mediated apoptotic signaling in Wistar rats. Circulation Research 397(1): 423–435. https://doi.org/10.1007/s00210-023-02620-4 [PubMed] [PMC]
Antonucci S, Di Sante M, Tonolo F, Pontarollo L, Scalcon V, Alanova P, Menabò R, Carpi A, Bindoli A, Rigobello MP, Giorgio M, Kaludercic N, Di Lisa F (2021) The determining role of mitochondrial reactive oxygen species generation and monoamine oxidase activity in doxorubicin-induced cardiotoxicity. Antioxidants & Redox Signaling 34(7): 531–550. https://doi.org/10.1089/ars.2019.7929 [PubMed] [PMC]
Awad HH, El-Derany MO, Mantawy EM, Michel HE, El-Naa MM, Salah El-Din RA, El-Brairy AI, El-Demerdash E (2021) Comparative study on beneficial effects of vitamins B and D in attenuating doxorubicin induced cardiotoxicity in rats: Emphasis on calcium homeostasis. Biomedicine & Pharmacotherapy 140: 111679. https://doi.org/10.1016/j.biopha.2021.111679 [PubMed]
Bauer G (2017) siRNA-based analysis of the abrogation of the protective function of membrane-associated catalase of tumor cells. Anticancer Research 37(2): 567–581. https://doi.org/10.21873/anticanres.11350 [PubMed]
Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S (2006) Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Molecular Pharmacology 70(6): 1844–1849. https://doi.org/10.1124/mol.106.030833 [PubMed]
Danilenko LM (2018) Doxorubicin-associated cardiomyopathy: New approaches to pharmacological correction using 3-(2,2,2-trimethylhydrazinium) propionate derivatives. Research Results in Pharmacology 4(1): 81–86. https://doi.org/10.3897/rrpharmacology.4.25530
Danilenko LM, Pokrovsky MV, Dovgan AP, Kolesnichenko PD, Timokhina AS, Kotelnikova AS (2018) Cardioprotective effects of derivatives of heterocyclic amino acids and 5-hydroxynicotinic acid in doxorubicin-induced cardiomyopathy. Regional Blood Circulation and Microcirculation 17(1): 90–96. https://doi.org/10.24884/1682-6655-2018-17-1-90-96
Gille A, Bodor ET, Ahmed K (2008) Nicotinic acid: pharmacological effects and mechanisms of action. Annual Review of Pharmacology and Toxicology 48: 79–106. https://doi.org/10.1146/annurev.pharmtox.48.113006.094746 [PubMed]
Gnaiger E, Kuznetsov AV (2002) Mitochondrial respiration at low levels of oxygen and cytochrome C. Biochemical Society Transactions 3(2): 252–258. [PubMed]
Hadigan C, Liebau J, Torriani M, Andersen R, Grinspoon S (2006) Improved triglycerides and insulin sensitivity with 3 months of acipimox in human immunodeficiency virus-infected patients with hypertriglyceridemia. The Journal of Clinical Endocrinology and Metabolism 91(11): 4438–4444. https://doi.org/10.1210/jc.2006-1174 [PubMed] [PMC]
Hsieh PL, Chu PM, Cheng HC, Huang YT, Chou WC, Tsai KL, Chan SH (2022) Dapagliflozin mitigates doxorubicin-caused myocardium damage by regulating akt-mediated oxidative stress, cardiac remodeling, and inflammation. International Journal of Molecular Sciences 23(17): 10146. https://doi.org/10.3390/ijms231710146 [PubMed] [PMC]
Kong CY, Guo Z, Song P, Zhang X, Yuan YP, Teng T, Yan L, Tang QZ (2022) Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. International Journal of Biological Sciences 18(2): 760–770. https://doi.org/10.7150/ijbs.65258 [PubMed] [PMC]
Ma Y, Yang L, Ma J, Lu L, Wang X, Ren J, Yang J (2017) Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochimica et Biophysica Acta. Molecular Basis of Disease 1863(8): 1904–1911. https://doi.org/10.1016/j.bbadis.2016.12.021 [PubMed]
Mao M, Zheng W, Deng B, Wang Y, Zhou D, Shen L, Niku W, Zhang NP (2023) Cinnamaldehyde alleviates doxorubicin-induced cardiotoxicity by decreasing oxidative stress and ferroptosis in cardiomyocytes. PLoS One 18(10): e0292124. https://doi.org/10.1371/journal.pone.0292124 [PubMed] [PMC]
Mateuszuk Ł, Campagna R, Kutryb-Zając B, Kuś K, Słominska EM, Smolenski RT, Chlopicki S (2020) Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochemical Pharmacology 178: 114019. https://doi.org/10.1016/j.bcp.2020.114019 [PubMed]
Neves MF (2023) Renin-angiotensin system inhibition and beta blockade adrenergic may be useful to attenuate cardiotoxicity by anthracyclines. Arquivos Brasileiros de Cardiologia 120(5): e20230280. https://doi.org/10.36660/abc.20230280 [PubMed] [PMC]
Numata G, Takimoto E (2023) A pacing-controlled procedure for the assessment of heart rate-dependent diastolic functions in murine heart failure models. Journal of Visualized Experiments 21: (197). https://doi.org/10.3791/65384[PubMed]
Pan JA, Zhang H, Lin H, Gao L, Zhang HL, Zhang JF, Wang CQ, Gu J (2021) Irisin ameliorates doxorubicin-induced cardiac perivascular fibrosis through inhibiting endothelial-to-mesenchymal transition by regulating ROS accumulation and autophagy disorder in endothelial cells. Redox Biology 46: 102120. https://doi.org/10.1016/j.redox.2021.102120[PubMed] [PMC]
Peresypkina A, Pazhinsky A, Danilenko L, Lugovskoy S, Pokrovskii M, Beskhmelnitsyna E, Solovev N, Pobeda A, Korokin M, Levkova E, Gubareva V, Korokina L, Martynova O, Soldatov V, Pokrovskii V (2020) Retinoprotective effect of 2-ethyl-3-hydroxy-6-methylpyridine nicotinate. Biology 9(3): 45. https://doi.org/10.3390/biology9030045 [PubMed] [PMC]
Ping Z, Fangfang T, Yuliang Z, Xinyong C, Lang H, Fan H, Jun M, Liang S (2023) Oxidative stress and pyroptosis in doxorubicin-induced heart failure and atrial fibrillation. Oxidative Medicine and Cellular Longevity 2023: 4938287. https://doi.org/10.1155/2023/4938287 [PubMed]
Qi W, Boliang W, Xiaoxi T, Guoqiang F, Jianbo X, GangW (2020) Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomedicine & Pharmacotherapy 122: 109547. https://doi.org/10.1016/j.biopha.2019.109547 [PubMed]
Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U (2021) Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomedicine & Pharmacotherapy 139: 111708. https://doi.org/10.1016/j.biopha.2021.111708 [PubMed]
Shabalala S, Muller CJ, Louw J, Johnson R (2017) Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Science 180: 160–170. https://doi.org/10.1016/j.lfs.2017.05.003 [PubMed]
Shcheblykina OV, Shcheblykin DV, Trunov KS, Danilenko AP, Lipatov VS (2022) Experimental study of new derivatives of 3-hydroxypyridine as pharmacological agents for the correction of ischemic brain injury after intracerebral hemorrhage. Research Results in Pharmacology 8(1): 71–83. https://doi.org/10.3897/rrpharmacology.8.80378
Shi S, Chen Y, Luo Z, Nie G, Dai Y (2023) Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy. Cell Communication and Signaling 21(1): 61 https://doi.org/10.1186/s12964-023-01077-5 [PubMed] [PMC]
Stetinová V, Grossmann V (2000) Effects of known and potential antioxidants on animal models of pathological processes (diabetes, gastric lesions, allergic bronchospasm). Experimental and Toxicologic Pathology 52(5): 473–479. https://doi.org/10.1016/S0940-2993(00)80087-1 [PubMed]
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Analytical Biochemistry 1(524): 13–30. https://doi.org/10.1016/j.ab.2016.10.021 [PubMed]
Vitale R, Marzocco S, Popolo A (2024) Role of oxidative stress and inflammation in doxorubicin-induced cardiotoxicity: a brief account. International Journal of Molecular Sciences https://doi.org/10.3390/ijms25137477 [PubMed]
Wallace KB, Sardão VA, Oliveira PJ (2020) Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circulation Research 126(7): 926–941. https://doi.org/10.1161/CIRCRESAHA.119.314681 [PubMed] [PMC]
Wei S, Ma W, Jiang C, Liu J, Liu J, Zhang B, Li W (2023) Hyperoside prevents doxorubicin-induced cardiotoxicity by inhibiting NOXs/ROS/NLRP3 inflammasome signaling pathway. Phytotherapy Research 37(9): 4196–4209. https://doi.org/10.1002/ptr.7900 [PubMed]
Wu J, Gao L, Fan H, Liu D, Lin M, Zhu M, Deng T, Song Y (2022) Calcium overload or underload? The effects of doxorubicin on the calcium dynamics in guinea pig hearts. Biomedicines 10(9): 2197. https://doi.org/10.3390/biomedicines10092197 [PubMed] [PMC]
Xie C, Zhou X, Liang C, Li X, Ge M, Chen Y, Yin J, Zhu J, Zhong C (2021) Apatinib triggers autophagic and apoptotic cell death via VEGFR2/STAT3/PD-L1 and ROS/Nrf2/p62 signaling in lung cancer. Journal of Experimental & Clinical Cancer Research 40(1): 266. https://doi.org/10.1186/s13046-021-02069-4 [PubMed] [PMC]
Zhang Q, Zhang Y, Xie B, Liu D, Wang Y, Zhou Z, Zhang Y, King E, Tse G, Liu T (2023) Resveratrol activation of SIRT1/MFN2 can improve mitochondria function, alleviating doxorubicin-induced myocardial injury. Cancer Innovation 2(4): 253–264. https://doi.org/10.1002/cai2.64 [PubMed] [PMC]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2024 Yana V. Loboda, Michael V. Pokrovskii, Mikhail V. Korokin, Alexey V. Kuznetsov, Tatiana N. Malorodova, Anton P. Danilenko, Anna A. Peresypkina, Oleg S. Gudyrev, Olga A. Kopeva, Tatyana G. Pokrovskaya, Liliya V. Korokina, Valeriya N. Khruslova, Valeriya A. Nazarenko, Tatyana V. Avtina, Alexey V. Deikin, Aleksandr A. Dolzhikov, Tatiana V. Puzanova, Lyudmila M. Danilenko

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

